¡Advertencia! El contenido en esta página está disponible solo en los siguientes idiomas: Inglés
How to choose a solution to handle the daily workflow in a Nuclear Medicine Department or Cyclotron Facility.
There are a variety of systems that are used to manage the daily workflow, but some are more suitable than others, depending on the problems you are facing and the environment in which you operate. To guide you in your selection, we have listed below three sets of problems you might be dealing with, along with the alternative solutions and the preferred solution.
(1)
PROBLEMS
- You need radiopharmaceuticals for examination purposes (e.g. PET/CT scan, Gamma spectroscopy) but you do not want to be bothered with strict regulation regarding manufacturing and preparation of radiopharmaceuticals
- Due to quality and cost reduction requirements, preparation of patient syringes and daily work in the nuclear medicine department must rise to a higher level
- You do not want to enter patient examinations and calculate injection doses manually in the IRIS automated injection device
- You need a system for inventory, waste, planning and order management, and you do not want to do the administration manually
- Regulation related to isotope bookkeeping enforces you to take action
- You are looking for a powerful and proven solution that fits your requirements and your limited budget.
POSSIBLE ALTERNATIVES
- Manual process that does not use a validated software package, with all activities written down on paper
- Different systems that can individually record the process data
- Workflow management software
BEST SOLUTION
IBC CLINIC management software

SOLUTION BENEFITS
- With IBC CLINIC, you have a software system that exactly fits your needs: isotope bookkeeping, inventory management, patient examination planning, radiopharmaceutical order management and injection registration
- The software ensures a closed loop from examination planning to injection
- You get the flexibility of preparing syringes from a multi-dose vial
- Quality will improve due to consistent guidance by the software of your work processes
- No transcription errors can occur due to the electronic link (DICOM, HL7) with RIS/PACS and HIS
- It supports Injection registration, including measurement and calculation of injected dose
- It automatically calculates doses (based on EANM dosage card) prior to ordering or preparing syringes
- It has a seamless and wireless bidirectional connection with the COMECER IRIS injection system.
WHY THE OTHER ALTERNATIVES ARE LESS SUITABLE
You have to perform many errors-prone manual calculations, for example to calculate the activity at patient examination time and, in case you use decay tables, the determined radioactivity is not accurate.
Due to regulation, you also have a manual register of radioactivity on stock and in waste. Moreover, you also need to handle a lot of paperwork to keep healthcare authorities satisfied during an on-site audit.
Different systems make it difficult to create reports and combine data because they don’t communicate with one another or the communication process requires a huge effort.
(2)
PROBLEMS
- You need radiopharmaceuticals for patient examination purposes (e.g. PET/CT scan, Gamma spectroscopy) and you are bothered with strict regulation regarding manufacturing and preparation of radiopharmaceuticals
- Due to GMP and Title 21 CFR Part 11, you are forced to improve the quality of your working processes for your radiopharmaceutical preparation and related working processes. You need to standardize your work and be able to prove that all actions related to material handling are carried out correctly
- You do not want to enter patient examinations and calculate injection doses manually
- You need a system for inventory, waste, planning and order management, and you do not want to handle the administration manually.
POSSIBLE ALTERNATIVES
- Manual process that does not use a validated software package, with all activities written down on paper
- Different systems that can individually record the process data
- Workflow management software
BEST SOLUTION
IBC Nuclear Medicine management software

SOLUTION BENEFITS
- You ensure high and consistent quality of the radiopharmaceuticals that you prepare. The preparations are based on user-definable protocols
- The software ensures a closed loop from examination planning to injection
- You save time and reduce transcription errors due to electronic links with RIS/PACS and HIS, based on DICOM or HL7
- You get a seamless connection with COMECER dispensing systems and with the IRIS automated injection system
- It supports injection registration, including measurement and calculation of injected dose
- Dose calculations are done automatically (based on EANM dosage card) prior to ordering or preparing syringes
- Inventory and waste management, planning, order management and injection registration are included as a default
- IBC Nuclear Medicine software can be upgraded to IBC GMP Radiopharmacy if you:
- want to improve your quality, traceability and GMP compliance
- want to improve your risk management
- wish to work more efficiently in your own production facility with cyclotron
- are forced by regulation, because you are going to ship your radiopharmaceuticals to hospitals and clinics in your area.
If you upgrade from IBC Nuclear Medicine, the functionality needed for your own nuclear medicine department for patient examination management, RIS/PACS link and injection registration remain at your disposal in the IBC GMP Radiopharmacy software.
WHY THE OTHER ALTERNATIVES ARE LESS SUITABLE
You have to perform many errors-prone manual calculations, for example to calculate the activity at patient examination time and, in case you use decay tables, the determined radioactivity is not accurate.
Due to regulation, you also have a manual register of radioactivity on stock and in waste. Moreover, you also need to handle a lot of paperwork to keep healthcare authorities satisfied during an on-site audit.
Different systems make it difficult to create reports and combine data because they do not communicate with one another or the communication process requires a huge effort.
(3)
PROBLEMS
- You have a production facility for radiopharmaceuticals and you are looking for one system to fulfil your needs regarding order management, production, document management, quality control, equipment management and shipment of radiopharmaceuticals
- You have to comply with GMP, title 21 CFR part 11
- You have to build your electronic batch records and want to manage that electronically, based on your own master production and control records, but don’t want to lose flexibility while you remain GMP compliant
- You need transport documents when shipping ordered radiopharmaceutical products to your customers. Documentation has to comply with ADR or other transport regulation
- Due to the strict requirements for registration of all production related activities, you have to enter all data in several information management systems. You dream of using a single management system with a single entry point for all production-related data
- You want to get rid of extensive paper records – you even aim for a paperless working environment.
POSSIBLE ALTERNATIVES
- Manual process that does not use a validated software package, with all activities written down on paper
- Different systems that can individually record the process data
- Workflow management software
BEST SOLUTION
IBC GMP Radiopharmacy management software
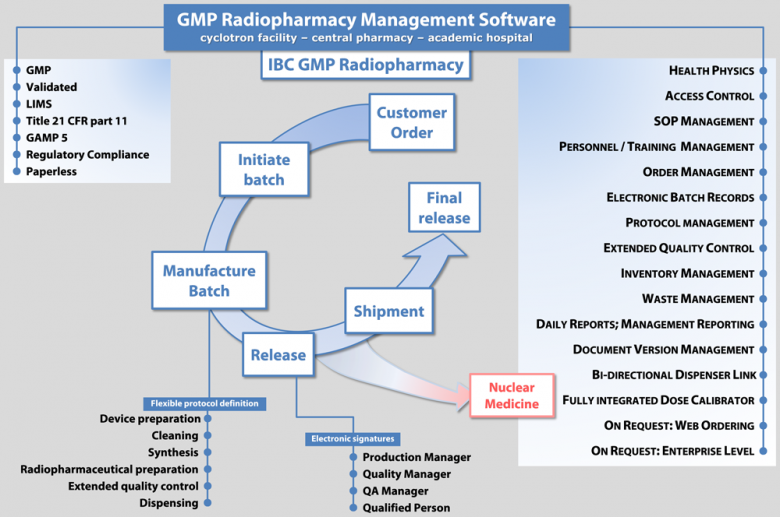
SOLUTION BENEFITS
- IBC GMP Radiopharmacy software reduces cost and improves efficiency and risk management due to single point of data entry and electronic traceability
- It combines flexibility with GMP compliance
- You can configure your way of working according to your standard operating procedures
- It gives you a single system for document and SOP management, training files, order management, master production and control records, batch records, inventory and waste management, quality control
- You save enormous amounts of time and reduce data entry errors due to the high integration of different processes related to radiopharmaceutical manufacturing – you can get rid of third-party tools that forced you to enter the same data multiple times to build your batch records
- It offers seamless integration with COMECER dispensing systems
- It is suitable for customers shipping radiopharmaceuticals to clients.
WHY THE OTHER ALTERNATIVES ARE LESS SUITABLE
Due to GMP regulation, you are forced to register a lot of data that, with the alternatives, has to be entered multiple times in different systems or on paper. You have to perform many errors-prone manual calculations, for example to calculate the activity at patient examination time and, in case you use decay tables, the determined radioactivity is not accurate.
All data related to your batch manufacturing gets printed in order to be able to combine it into a single batch record. You have problems in managing your backup because you have a giant paper archive. Moreover, you also need to handle a lot of paperwork to keep healthcare authorities satisfied during an on-site audit. You get lost in case of a recall or in case of time pressure during release of a batch.
Standard operating procedures are printed and available in different rooms. Change management to these documents is a lot of work, as physical documents must be replaced (while a validated software system can present relevant documents at the right moment as well).
Different systems can help you to register the data you need to comply with the GMP directives, but they make it difficult to create reports and combine data because they do not communicate with one another or the communication process requires a huge effort.
Improving Nuclear Medicine Workflow!
Learn more about the IBC-CLINIC Management software >>
Learn more about the IBC-NUCLEAR MEDICINE Management software >>
Learn more about the IBC-GMP RADIOPHARMACY Management software >>





