- Products
- Video
- Events
- News
- Landing
- Pages
Avertissement ! Le contenu de cette page est disponible uniquement dans les langues suivantes: Anglais - Italien

An aseptic industrial process can be conducted inside an isolator. Isolators are machines equipped with high efficiency air filters, which create a controlled environment: they protect both the handled material and the operator, eliminating direct contact between the inside/outside and all the related risks. They can be decontaminated through a validated cycle: the purpose of this article is to provide the reader with a series of ideas and reflections on optimising a cycle through VPHP (Vapour Phase Hydrogen Peroxide).
A validated decontamination method must be able to reduce the microbiological charge to the preset requirement, usually referred to as a SAL (Sterility Assurance Level) of 6log. There is a wide range of sterilising substances on the market, each with special characteristics, but hydrogen peroxide solution has a number of features that make it beneficial: it has a clear and proven sporicidal effect on a broad spectrum of microbial agents already at a low concentration and degrades into non-toxic by-products.
For the sterilisation of isolators since the ‘80s onwards the use of peroxide has become increasingly popular becoming the decontamination system of “excellence”. The effectiveness/optimisation of the process depends on several factors, in particular on:

In any case there are some general aspects to be considered. In this article, we draw attention to 4 of these aspects:
The isolator provides a physical barrier between a process and the external environment. In standard isolator ventilation conditions, the inlet air crosses the HEPA (High Efficiency Particulate Air Filter) filters and enters the operating chamber. To go out it crosses other filters and enters the plenum where it is channelled into the extraction ducts. Due to this ventilation, there are two ways of introducing peroxide into the chamber: ‘injection in plenum’ where the VPHP ducts are connected to the ventilation and ‘direct injection’ where instead they are connected directly to the chamber.

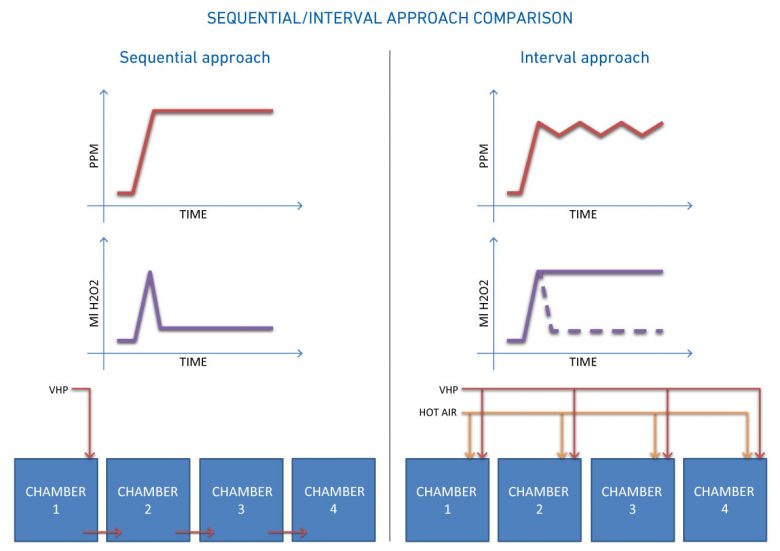
When trying to use a single generator to decontaminate several isolated contexts, the main purpose is to save both in terms of infrastructure and in overall cycle time. The approach that can be followed is that of a sequential or interval system.

A generator used in a sequential manner is operated on average less than 10% in the overall cycle time: the total cycle will be the sum of all the individual cycle times. While in the interval approach, the generator is used 100% of the time, with a HOT AIR-VPHP alternate time approach, where the concentration rises evenly between the various chambers, compensating the volumes through time intervals and air and temperature flows: the total cycle time becomes equal to the cycle time of a chamber.
Hydrogen peroxide solution is a sterilising agent that can be used in dry vapour, damp or ionized vapour form. Each method uses a different application to transform the peroxide into vapour and/or micro-drops.
Description: Dry hydrogen peroxide vapour is obtained by injecting the liquid over a heated plate, subjected to a hot air flow, which acts as a carrier to the chamber. This way a flash vaporisation is obtained, where water and peroxide are vaporised at the same time.
Pros and Cons: The vapour can be generated in a remote position with respect to the chamber and conveyed easily into the ventilation. It requires pre-conditioning with reduction of relative air humidity. The vapour phase of the sterilising agent ensures good internal diffusion, reaching even the most unfavourable locations (edges, cracks…). Crosses LAF filters more easily than a wet approach. Avoiding condensation on the surfaces is theoretically less aggressive on the materials. It requires a longer cycle, but in a real load context, multi-chamber situations or ventilation phases with limited flows, it is still very effective.
Description: The vapour is introduced into the chamber becoming micro-condensation: invisible small drops are formed on all surfaces.
Pros and Cons: Since the micro-condensation will have a greater concentration of H2O2 it will be faster in decontamination than dry vapour. It avoids the preliminary dehumidification phase, since it does not have to reduce the relative air humidity. It is more difficult to transport without incurring in macro-condensation inside the ventilation circuit and even distribution is more difficult. By saturating the air with water and H2O2 the ventilation phase is longer and injection through HEPA filters is longer.
Description: Ionization produces radicals that improve the action of the peroxide and act quickly against contamination. Special nozzles create a fine mist, containing a high concentration of species reactive to oxygen. Using ionizing injectors, the liquid is therefore introduced in the form of micro-drops activated directly in the chamber.
Pros and Cons: It cannot be transported in atomized form and therefore requires injection into the chamber. It is very effective at low concentrations with the possibility of very fast cycles, but requires careful positioning and sizing of the nozzles.
All the aspects analysed above affect the individual phases of the cycle, which usually consists of 4 phases: dehumidification, conditioning, decontamination and ventilation.

This stage involves the creation of the initial conditions required for decontamination, adjusting the relative humidity by using a dehumidifier.
Optimisation factors:
During this stage, the concentration of peroxide within the isolator necessary to ensure the “killing” effect is reached.
In the DRY and WET approach:
In the IONIZED approach:
The phase is very fast in any operating mode.
In this phase the concentration of H2O2 is kept stable for all the time necessary to reach the desired SAL value. The effectiveness of the cycle is normally verified using performance indicators, both chemical (CIs) and biological (BIs). The BIs are placed in the chamber and are decontaminated. At the end of the cycle the BIs are collected and incubated to verify the absence of microbiological growth, proving the effectiveness of the decontamination cycle.
It is the most critical phase because it is necessary to understand when the SAL is reached, and it makes no sense to assess the related times without considering:

Load distribution example inside a glovebox
The variation over time of the outside temperature and initial humidity of the room are parameters that are difficult to control and predict.
Once the work chamber has been sterilised, the concentration of peroxide, expressed in ppm (parts per million), must return below the TLV (Threshold Limit Value) generally corresponding to 1ppm, as indicated by the OSHA (Occupational Safety and Health Administration). This is the phase that mostly affects the cycle times, because it is directly proportional to the air flows, depends strongly on the isolator and may take a few minutes to several hours.
Optimisation factors:
Each phase of the cycle can therefore be subject to time optimisation, but a cycle that is unstable or subject to atypical phenomena should not be validated. Excessively short cycle times may lead to the appearance of phenomena such as false positives/negatives or slow release (de-absorption) of the VPHP by the Bis coating material, which could prolong the killing action. At the end of the cycle, the BI is impregnated, continuing to undergo the effects of the trapped VHP and its actual decontamination ends several minutes after the end of the «nominal» cycle in the chamber, including the time to extract it from the chamber and incubate it. An additional risk is the validation of a process with unstable thermal parameters: different humidity and initial temperature conditions may cause discrepancies during the conditioning and decontamination phase. In addition, the process must ensure the desired SAL: the use of BIs with excessively low D-value, the use of a minimal load during validation and underestimation of the decontamination time safety factor, can lead to a very short cycle but with a statistically insignificant result. Finally, during validation, it is necessary to check that there are no residues of VPHP over 1ppm in the chamber, at the end of cycle.
Decontamination by means of VPHP is a process that depends on many factors and therefore it is not possible to define a “standard cycle” in advance: in fact, the characteristics of the cycle and the approach to be followed depend intrinsically on the isolator and environmental conditions.
It is therefore a process to be optimised as required, case-by-case, using different technologies and always trying to optimise its performance, avoiding the potential risks.
Connectez-vous à votre compte Comecer pour télécharger les fiches techniques
Log In Lost PasswordActualités et invitations à des événements et à des salons directement dans votre messagerie
S'inscrire

