- Products
- Video
- Events
- News
- Landing
- Pages
Gallium-68-based tracers are more and more often used in nuclear medicine departments in Europe and around the world both for PET/CT tests to study neuroendocrine tumours and in all tests linked with the study of somatostatin receptors.
This trend is due to the flexibility of Gallium-68 in the type of chemical bonding, availability of 68Ge/68Ga generators and a good relationship between the dose administered and the decay times.
In the past 4 years, the number of scientific publications on this topic has increased significantly, as well as the number of patients treated with this type of radiopharmaceutical. Publication of detailed studies in the European Pharmacopoeia and “market authorisation” for certain 68Ga generators are breaking down the barriers that made it complex to implement this radiotracer in routine preparations.
However, there are still some critical aspects that may hinder diffusion of this radiotracer. Automatic synthesis modules must be used in the production process to manufacture Gallium-68, it requires much more complex Quality Control systems compared to what is normally found in small contexts of hospital nuclear medicine, and requires “synthesis and dispensing” work flow management inside hot cells according to the regulations, whether country-specific or European. The required premise is that production of radiopharmaceuticals bearing Gallium-68 marking is influenced by multiple factors. Synthesis is integrated in a hospital context that changes each time according to how the pharmaceutical risk is managed and how the preparation standards are interpreted. From this point of view, different solutions can be equally adequate. However, GMP guidelines are also getting involved in this context and will, over time, make regulatory bodies and control agencies standardise the requirements of the process developed in hospitals to those of the process developed in centralised radiopharmacies.
How does this approach translate into actual operations, and what type of equipment investment does handling and preparation involve for those who want to introduce use of this radioisotope in their routine activity?
To answer this question, one must first analyse how this isotope enters a hospital ward today and how it can do so in the future. In fact, there are three different ways:
Today, the first one prevails. However, the option of centralised distribution, especially with EU and FDA marketing authorisation with regard to 68Ga-DOTA-x (or similar), will become a tangible and usable option in the near future. On the other hand, and despite being in its initial stages, cyclotron production enjoys the potential of becoming the most attractive in terms of costs by avoiding the purchase of generators and integrating within the normal production flow of cyclotron radiotracers. A tangible example is the recent technology introduced by IBA for Gallium-68 production by liquid targets.
Therefore, a handling, synthesis or dispensing cell must consider all three aspects for an investment to be made and be valuable in coming years.
Once Gallium-68, enters a nuclear medicine department, the various operating methods can be assessed, which will lead to the final phase of the patient’s treatment.
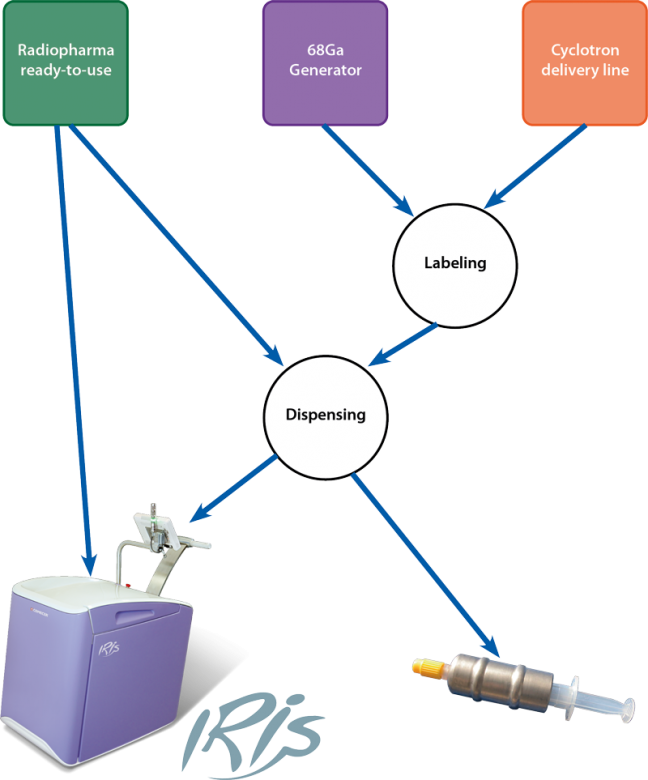
Neglecting the Quality Control phase, which still cannot be omitted except for medicines sold within marketing authorisation, the patient can be treated in two ways: by means of injection with a traditional syringe prepared in an aseptic area, or via an automatic injection system like our IRIS multi-dose injector, which can inject isotopes such as Gallium-68 and not only Fluoro-18.
Using the IRIS system, it is possible to bypass the preparation and labelling phases, using a multi-dose vial ready to be injected and distributed by a centralised pharmacy. The operating flow would be identical to what is used by many premises today for FDG.
IRIS can also be used with a multi-dose vial produced in-house. Its application reduces operator radio exposure when transporting the radiopharmaceutical or during injection procedures. The operating flow for preparation is also quicker since only multi-dose vials and Quality Control vials are removed from the hot cell. Syringes are therefore not handled.
When production starts from the Gallium-68 generator, which is in the majority of cases today, there are two possible operating flows that are completely different from a standard point of view. These flows require different types of hot cells since they require different types of classified areas.

In the former case, the generator, the synthesis and dispensing (if applicable) modules are inserted in a single shielded and classified compartment, whereas in the latter they are inserted in distinct and separate compartments.
It is not possible to use a “cold kit” to label with 68Ga (although it is possible that new technologies in this regard will be launched on the market in coming years), and an elution process is required from the generator with an HCI solution and very complex pumping systems compared to 99mTc generators. These operations are therefore considered a “high microbiological risk” on a regulatory level, and local authorities often impose the use of a Class A LAF (laminar flow) isolator for aseptic processes.
Therefore, the use of Class A hoods is often not accepted in many countries. From a radioprotection point of view, it is also a choice that safeguards the operator more, as by its nature the hood only partially shields. Since Gallium-68 is an isotope for PET investigations and it also has high-energy components in the spectrum of decay, its preparation is critical from a viewpoint of operator protection against ionising radiation.
For this reason and the regulatory aspect, a Class A isolator remains the primary choice for this type of process.
GMP classification of the Class A work area is an obligation on two fronts: first, it has specific limits with regard to the particulate and the presence of microbiological contamination. Moreover, Class A is only one of the requirements that form part of a much larger request or need to carry out an aseptic process. It is therefore more correct to say that it is not only necessary to have a “Class A cell”, but also a system that implements an aseptic process as a whole.
This has several implications, the most important of which is: is it possible to carry out an aseptic process in an isolated compartment in which an automatic synthesis system, a 68Ga generator, a number of waste vials, chemical reagents, disposable complexes to unpack, a dispenser, end containers and accessories are positioned at the same time?
It certainly is very complex and perhaps difficult to accept by one with an attentive eye to the regulations. As a GMP process it is definitely not acceptable, so much so that it has been established that synthesis and dispensing must be separated in production processes of any tracers.
In a chemical context, a “closed disposable” is often considered as an extenuation, since it is closed and there is no possible external contamination. However, this approach has various critical points:
If it is currently already difficult to accept the regulatory approach, separating the two areas remains the only safe, long-term solution.

THE COMECER SOLUTIONS
With its experience in the radiopharmaceutical industry, Comecer offers solutions to manage the production, handling and dispensing process of the product, in accordance with the highest standards in terms of compliance with regulations, usability and investment.
Comecer offers different types of hot cells and dispensers that enable the workflow described in this article.
MUSA 68Ga: class A shielded isolator, a solution for medium-low activity, compact, gloved hand passage doors, production of multi-dose vial or syringes for IRIS

BBST-COMBO: shielded isolator for synthesis and dispensing (class A), a solution for high activity, tele-pliers for dispensing, production of multi-dose vial or vials for IRIS

ELENA: Class A shielded isolator, a solution for medium-low activity, high ergonomics, gloved hand passage doors, production of multi-dose vial or syringes for IRIS
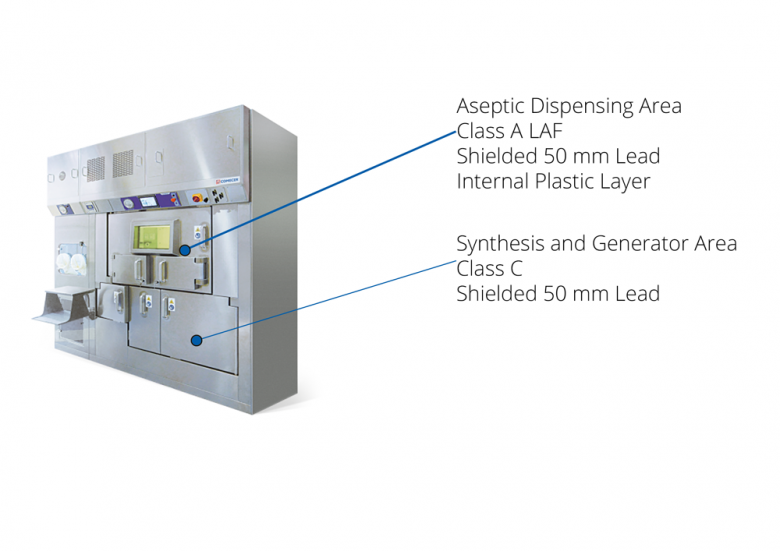
BBS: shielded cell for synthesis, a solution for high activity and productivity, it must be connected to a shielded isolator for dispensing

This comparison table helps asses what kind of solution is most suited to specific needs and operating requirements without sacrificing the concepts discussed:
| CHARACTERISTIC | MUSA 68Ga | ELENA | BBST-COMBO | BBS |
| Option of two separate compartments, synthesis in Class C and dispensing in Class A | Yes | Yes | Yes | Synthesis only |
| Option to have synthesis and dispensing in the same Class A compartment | Yes | Yes | No | No |
| Primary cell characteristic | Compact | Highly ergonomic structure (increased Class A width area and raised synthesis module position) | High activity processes managed by tele-pliers | Ideal for synthesis in large laboratories |
| Option to insert the cell in a GMP process | Yes | Yes | Yes | Yes |
| Ideal for processes with beta emitters such as 177Lu and 90Y | Optional
beta shielding |
Included
beta shielding |
Beta shielded not requested since there are no direct manipulations | Beta shielded not requested since there are no direct manipulations |
| Option to have double Class B airlock for Class A material inlet and outlet | Yes | Yes | Yes | Not required |
| Automatic airlock lifter in order to load (in the case of a mother vial inlet) and unload (in the case of a multi-dose vial) heavy lead containers easily and with maximum ergonomics | Yes | Yes | Not required | Not required |
| Synthesis area able to contain one or more 68Ge/68Ga generators | Yes | Yes | Yes | Yes |
| Number of generators that can be installed | 2 | 2 | 2 | 2 |
| Connection to a possible cyclotron line | Optional | Optional | Present | Present |
| Option of anti-corrosion protection | Yes | Yes | Yes | Yes |
| Restrictions regarding type of modules that can be installed due to dimensions | Assessment required | Assessment required | Area suitable for large synthesis modules | Area suitable for large synthesis modules |
| Option to provide corollary HPLC to the module | No | Only if installed in Class A | Yes | Yes |
| Recommended dispensers for syringes | FEBO | FEBO | FEBO | Dispensing cell required |
| Recommended dispensers for vials | ARGO | ARGO | ARGO | Dispensing cell required |
| Ideal for production of a multi-dose vial that can be used for IRIS | Yes | Yes | Yes | Dispensing cell required for QC |
The anti-acid protection is considered an important option since it has been pointed out in various hospitals and radiopharmacies how elution of the generator with HCI solutions, the dynamics of synthesis and any small mistakes made by operators, are able to trigger corrosion processes, even on pharmaceutical steels such as AISI 316L commonly used in our isolators.
Corrosion processes can be salvaged, however they trigger problems with both the hot cell and the pharmaceutical. For this reason, Comecer always provides the option to implement effective countermeasures against corrosion of the inner walls of hot cells.
Consider also that Gallium-68 labelling with peptides is often accompanied by labelling with Lutetium-177 and Yttrium-90. In fact, synthesis modules address these isotopes with the same chemical processes, and at times with the same disposable. In order to maintain maximum flexibility and safety of the solution, it is thus necessary to provide shielding for beta emitters, such as in MUSA 68Ga and ELENA manipulation cells.
68Ga accompanies therapies based on 177Lu and 90Y. For this reason, while managing the preparation flow, it is important to consider that more and more often, the use of 68Ga for a diagnosis is preparatory to the next therapeutic treatment based on beta emitters. A hot cell with these characteristics is therefore ideal for 68Ga taken individually as well as for procedures that are indicated with the word “teragnostic”.
Comecer offers products that ensure their usage over time and are able to address regulatory issues, not only by considering the current demands, but also in preparation for future ones. Operating flexibility and the ability to adapt to different types of diagnostic or therapeutic treatment processes are key factors to maximise investment protection and obtain perfect results over time.
Do you have a technical question? Would you like to actually see IRIS with no obligation? Do you want to request a quote? Fill out the form below.
Log in to your Comecer Account to download data sheets
Log In Lost PasswordNews and invitations to events and fairs directly in your mailbox
Subscribe

