- Products
- Video
- Events
- News
- Landing
- Pages
TIMO 2 is a semi-automatic dispensing system that makes use of components and software developed and adopted by COMECER on ALTHEA PC automatic fractionators.
TIMO 2 dispensing system allows the fractionation of radiopharmaceuticals in single-dose syringes of PET and SPECT isotopes and for radiotherapy.
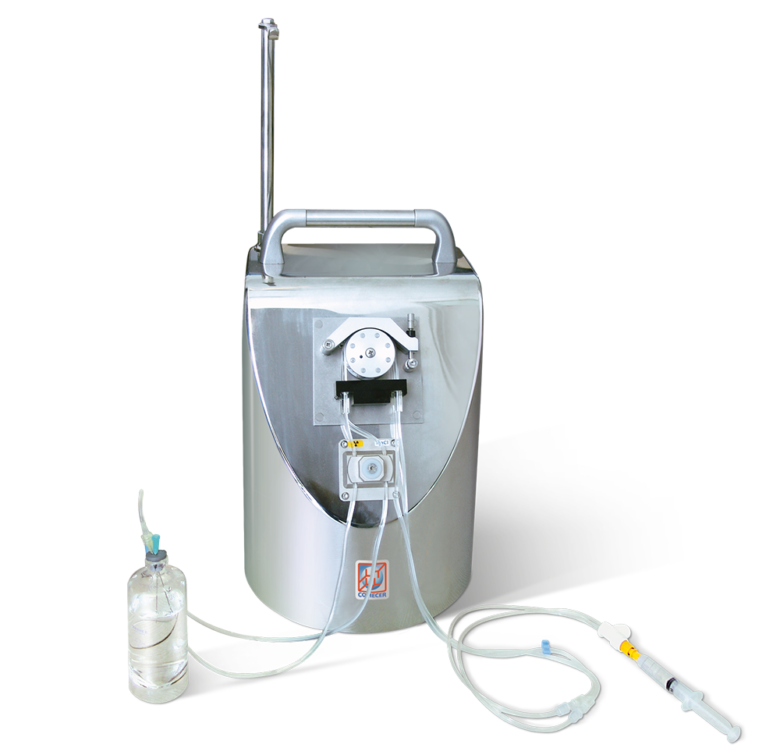
The system can be installed inside a shielded laminar flow isolator, thereby ensuring sterile radiopharmaceutical preparations.
The single-use set and the special patented pierceable septum allow packaging of the closed syringes ready to be transported to the administration unit, thereby protecting the sterility of the preparation.
The management software, combined with COMECER activity calibrators, allows for an easy and intuitive fractionation procedure and a quick preparation of the single-dose syringes. The filling system consists of a two-channel peristaltic pump. One channel is for the radiopharmaceutical, whereas the other is for the saline solution. By managing these two channels, the syringes can be filled with a defined amount of activity that can be diluted in saline solution until reaching the required volume.
The PC control panel allows monitoring of the filling process in real-time by the minute. The recipe management and other additional functions, such as the calculation of a certain activity required in the syringe at a certain time, can be obtained through the control software. Syringe fractionation starts from an external mother vial or from the radiopharmaceutical coming directly from the synthesis module. The special syringes allow for safe transport from a radioprotection and pharmaceutical point of view.
Its ease of use and versatility make the TIMO2 semi-automatic fractionator suitable to be used both as original equipment or as a back-up module. TIMO2 ensures a significant reduction of the dose the operator is exposed to while setting up the radiopharmaceutical dose. It can be installed in various cells and shielded isolators equipped with dose calibrator for activity dimensions. Its compact size and reduced weight, as well as the carrying handle, allow it to be easily removed from the cell for maintenance and cleaning operations.
All the production data are stored in a data logger on the management PC. At the end of cycle, the information of the production batch is stored in compliance with the cGMP and GAMP Standards.
The data logger records the following information:


